Elemental cycling in terrestrial mud volcanoes is affected by microbial communities and metabolic characteristics
Methane is one of the most potent greenhouse gases in Earth’s atmosphere. It traps 28 times more heat per mass unit than carbon dioxide over a 100-year period. Recent estimates have revealed that MVs and seepages onshore and offshore might account for one third of methane emissions from natural sources. Unlike their marine counterparts, whose emissions are mitigated by aerobic methane oxidation in seawater above them, terrestrial MVs emit methane directly into the atmosphere. Previous studies have indicated that the exact quantity of methane emission is primarily controlled by in situ microbial production and consumption near the surface. Although the fate of methane produced in situ has been demonstrated in previous studies, the assemblage and distribution of community members responsible for upstream organic mineralization and production of precursors (e.g., H2, acetate, and methyl-compounds) for methanogenesis in terrestrial mud volcano environments have not been fully revealed. Additionally, most terrestrial MVs are characterized by a limited availability of sulfate (<1 mM). Whether the syntrophic partnerships between ANME groups and sulfate reducing bacteria commonly observed in sulfate-rich marine settings could be maintained is not clear.
A research team at the Institute of Oceanography in National Taiwan University, led by Dr. Tzu-Hsuan Tu and Prof. Pei-Ling Wang, showed new findings for carbon cycling in terrestrial mud volcanoes. They collected sediment cores and muddy fluids in a terrestrial ferruginous, sulfate-depleted mud volcano (Lei-Hong-Hou, LGH mud volcano) in eastern Taiwan. Microbial communities and metagenomic data were obtained by Next Generation Sequencing technology. While porewater geochemistry including concentrations of chloride, total iron, methane, DOC, and DIC and carbon isotopic compositions of methane and DIC were measured, the interaction between microbial community structures and porewater geochemistry was clarified.
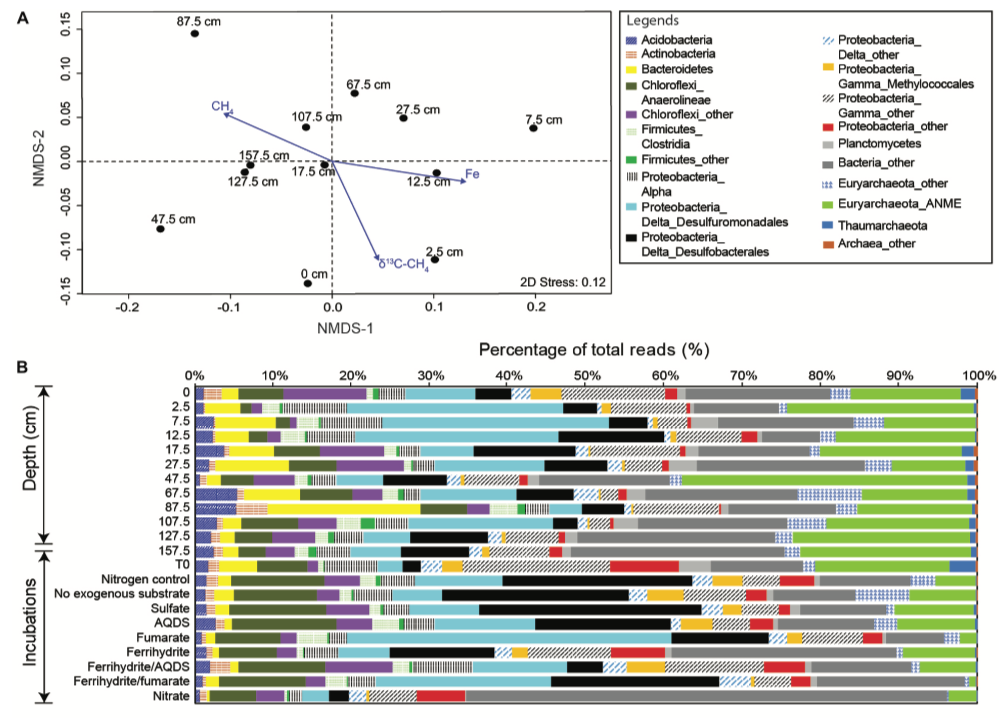
Geochemical profiles combined with 16S rRNA gene abundances indicated that anaerobic oxidation of methane (AOM) mediated by ANME-2a group coincided with iron/manganese reduction by Desulfuromonadales at shallow depths deprived of sulfate (Figure 1). Metagenomic analyses also revealed that functional genes for AOM and metal reduction, in particular intercellular electron transport were more abundant at shallow intervals. The distribution pattern of functional genes suggested potential intercellular interactions for electron transport involved in AOM. In contrast, genes responsible for methanogenesis and degradation of chitin and plant-derived molecules were more abundant at depth. Finally, genes responsible for aerobic methane oxidation were more abundant in the bubbling pool and near surface sediments.
Overall, our results demonstrated that various community members were compartmentalized into stratified niches along geochemical gradients. These community members form a metabolic network that cascades the carbon transformation from the upstream degradation of recalcitrant organic carbon with fermentative production of labile organic entities and methane to downstream methane oxidation and metal reduction near the surface. Such a metabolic architecture enables effective methane removal under ferruginous, sulfate-depleted conditions in terrestrial MVs.
Figure 1. Community compositions and variations revealed by 16S rRNA gene amplicons. (A) Non-metric multidimensional scaling of community relatedness. The results revealed that the δ13C values of methane and concentration of iron decreased with depth. In contrast, concentration of methane gradually increased to the core bottom. (B) Microbial community structure varied along depth. Desulfuromonadales (light blue) capable of reducing iron and manganese and ANME-2a group were much abundant at shallow depths.